Imaging Human Brain Function With Minimal Mobility Restrictions
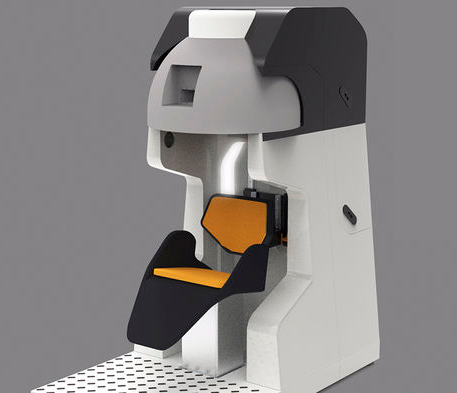 The Garwood lab is developing a next generation neuroimaging
platform based on magnetic resonance imaging (MRI). In this project,
we are designing, building, and testing the first-ever portable MRI
scanner that addresses all of the limitations and allows the magnet to
be brought to the subject rather than the other way around. To demonstrate
the value of this fundamental shift in experimental paradigm we will conduct
a pilot study involving motor planning which makes use of natural reaching
behaviors which are inaccessible with current human imaging technologies.
This first-of-its-kind MRI system will open new frontiers in human brain research,
allowing better understanding of human behavior and motor coordination. This work is
supported by NIH UO1 EB025153. (Image courtesy of Ben Parkinson)
The Garwood lab is developing a next generation neuroimaging
platform based on magnetic resonance imaging (MRI). In this project,
we are designing, building, and testing the first-ever portable MRI
scanner that addresses all of the limitations and allows the magnet to
be brought to the subject rather than the other way around. To demonstrate
the value of this fundamental shift in experimental paradigm we will conduct
a pilot study involving motor planning which makes use of natural reaching
behaviors which are inaccessible with current human imaging technologies.
This first-of-its-kind MRI system will open new frontiers in human brain research,
allowing better understanding of human behavior and motor coordination. This work is
supported by NIH UO1 EB025153. (Image courtesy of Ben Parkinson)
Desktop Scanner for Measuring pO2 in the Bioartificial Pancreas
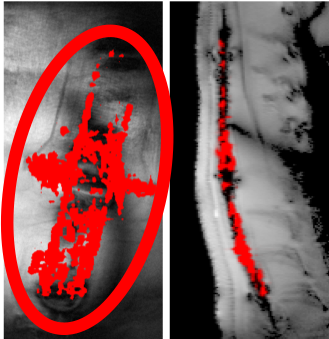 The Garwood lab is fabricating a compact, cost-efficient scanner – based on MRI technology
- to be used for making in-vivo oxygen measurements within islet macroencapsulation
devices. Islet replacement therapy is used to treat Type 1 Diabetes and has the
potential to allow these people to keep their blood sugar within healthy limits without
the need for any external insulin injection or monitoring. One major limitation of this
treatment is the need for life-long immunosuppression. Encapsulation of islets can remove
the need for immunosuppression but leads to oxygen starvation of the transplanted tissue. Our
scanner, in conjunction with an electrolysis based oxygen generator, can be used to
measure oxygenation within macroencapsulation devices, and then supply the optimal
levels of oxygen to the islets. This work is supported by the Schott Foundation, Minnesota Lions Diabetes Foundation, Olson Foundation.
The Garwood lab is fabricating a compact, cost-efficient scanner – based on MRI technology
- to be used for making in-vivo oxygen measurements within islet macroencapsulation
devices. Islet replacement therapy is used to treat Type 1 Diabetes and has the
potential to allow these people to keep their blood sugar within healthy limits without
the need for any external insulin injection or monitoring. One major limitation of this
treatment is the need for life-long immunosuppression. Encapsulation of islets can remove
the need for immunosuppression but leads to oxygen starvation of the transplanted tissue. Our
scanner, in conjunction with an electrolysis based oxygen generator, can be used to
measure oxygenation within macroencapsulation devices, and then supply the optimal
levels of oxygen to the islets. This work is supported by the Schott Foundation, Minnesota Lions Diabetes Foundation, Olson Foundation.
Non-Invasive Quantification of Iron Oxide Nanoparticles
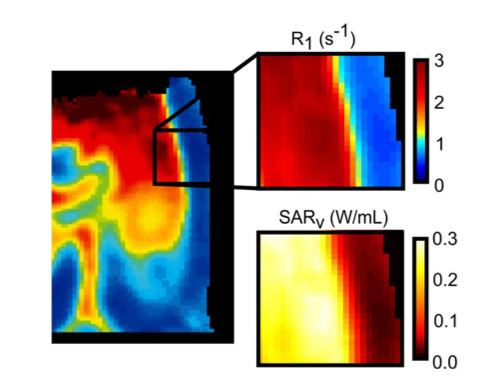 The Garwood lab is establishing echoless MRI as a non-invasive quantitative measurement of
iron-oxide nanoparticle (IONP) distribution within organ systems. Organ transplantation is
a life-saving treatment for patients suffering from a wide range of degenerative diseases
, but is limited due to low organ availability. The recent demonstration to reheat cryopreserved tissues
with IONPs has removed a major obstacle in the development of organ banking through cryopreservation
. Translating this new technology into organ systems requires quantitative information about
the IONP distribution throughout the organ, which we have demonstrated through the use of
R1 maps produced with swept imaging with Fourier transform (SWIFT). Better IONP quantification
will allow us to predict the heating produced by IONPs and accurately assess IONP distribution throughout
the organ. This work is supported by NIH R01 HL135046-01A1 & NIH R01 DK117425-01.
The Garwood lab is establishing echoless MRI as a non-invasive quantitative measurement of
iron-oxide nanoparticle (IONP) distribution within organ systems. Organ transplantation is
a life-saving treatment for patients suffering from a wide range of degenerative diseases
, but is limited due to low organ availability. The recent demonstration to reheat cryopreserved tissues
with IONPs has removed a major obstacle in the development of organ banking through cryopreservation
. Translating this new technology into organ systems requires quantitative information about
the IONP distribution throughout the organ, which we have demonstrated through the use of
R1 maps produced with swept imaging with Fourier transform (SWIFT). Better IONP quantification
will allow us to predict the heating produced by IONPs and accurately assess IONP distribution throughout
the organ. This work is supported by NIH R01 HL135046-01A1 & NIH R01 DK117425-01.
 The Garwood lab is developing a next generation neuroimaging
platform based on magnetic resonance imaging (MRI). In this project,
we are designing, building, and testing the first-ever portable MRI
scanner that addresses all of the limitations and allows the magnet to
be brought to the subject rather than the other way around. To demonstrate
the value of this fundamental shift in experimental paradigm we will conduct
a pilot study involving motor planning which makes use of natural reaching
behaviors which are inaccessible with current human imaging technologies.
This first-of-its-kind MRI system will open new frontiers in human brain research,
allowing better understanding of human behavior and motor coordination. This work is
supported by NIH UO1 EB025153. (Image courtesy of Ben Parkinson)
The Garwood lab is developing a next generation neuroimaging
platform based on magnetic resonance imaging (MRI). In this project,
we are designing, building, and testing the first-ever portable MRI
scanner that addresses all of the limitations and allows the magnet to
be brought to the subject rather than the other way around. To demonstrate
the value of this fundamental shift in experimental paradigm we will conduct
a pilot study involving motor planning which makes use of natural reaching
behaviors which are inaccessible with current human imaging technologies.
This first-of-its-kind MRI system will open new frontiers in human brain research,
allowing better understanding of human behavior and motor coordination. This work is
supported by NIH UO1 EB025153. (Image courtesy of Ben Parkinson) The Garwood lab is fabricating a compact, cost-efficient scanner – based on MRI technology
- to be used for making in-vivo oxygen measurements within islet macroencapsulation
devices. Islet replacement therapy is used to treat Type 1 Diabetes and has the
potential to allow these people to keep their blood sugar within healthy limits without
the need for any external insulin injection or monitoring. One major limitation of this
treatment is the need for life-long immunosuppression. Encapsulation of islets can remove
the need for immunosuppression but leads to oxygen starvation of the transplanted tissue. Our
scanner, in conjunction with an electrolysis based oxygen generator, can be used to
measure oxygenation within macroencapsulation devices, and then supply the optimal
levels of oxygen to the islets. This work is supported by the Schott Foundation, Minnesota Lions Diabetes Foundation, Olson Foundation.
The Garwood lab is fabricating a compact, cost-efficient scanner – based on MRI technology
- to be used for making in-vivo oxygen measurements within islet macroencapsulation
devices. Islet replacement therapy is used to treat Type 1 Diabetes and has the
potential to allow these people to keep their blood sugar within healthy limits without
the need for any external insulin injection or monitoring. One major limitation of this
treatment is the need for life-long immunosuppression. Encapsulation of islets can remove
the need for immunosuppression but leads to oxygen starvation of the transplanted tissue. Our
scanner, in conjunction with an electrolysis based oxygen generator, can be used to
measure oxygenation within macroencapsulation devices, and then supply the optimal
levels of oxygen to the islets. This work is supported by the Schott Foundation, Minnesota Lions Diabetes Foundation, Olson Foundation.
 The Garwood lab is establishing echoless MRI as a non-invasive quantitative measurement of
iron-oxide nanoparticle (IONP) distribution within organ systems. Organ transplantation is
a life-saving treatment for patients suffering from a wide range of degenerative diseases
, but is limited due to low organ availability. The recent demonstration to reheat cryopreserved tissues
with IONPs has removed a major obstacle in the development of organ banking through cryopreservation
. Translating this new technology into organ systems requires quantitative information about
the IONP distribution throughout the organ, which we have demonstrated through the use of
R1 maps produced with swept imaging with Fourier transform (SWIFT). Better IONP quantification
will allow us to predict the heating produced by IONPs and accurately assess IONP distribution throughout
the organ. This work is supported by NIH R01 HL135046-01A1 & NIH R01 DK117425-01.
The Garwood lab is establishing echoless MRI as a non-invasive quantitative measurement of
iron-oxide nanoparticle (IONP) distribution within organ systems. Organ transplantation is
a life-saving treatment for patients suffering from a wide range of degenerative diseases
, but is limited due to low organ availability. The recent demonstration to reheat cryopreserved tissues
with IONPs has removed a major obstacle in the development of organ banking through cryopreservation
. Translating this new technology into organ systems requires quantitative information about
the IONP distribution throughout the organ, which we have demonstrated through the use of
R1 maps produced with swept imaging with Fourier transform (SWIFT). Better IONP quantification
will allow us to predict the heating produced by IONPs and accurately assess IONP distribution throughout
the organ. This work is supported by NIH R01 HL135046-01A1 & NIH R01 DK117425-01.